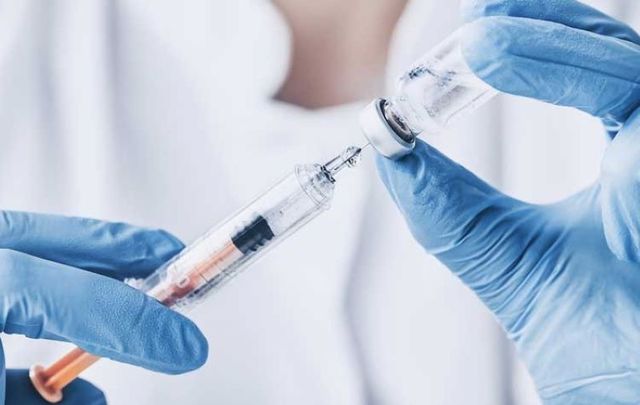
Northern Ireland has been selected to take part in a major UK trial of a potential COVID-19 vaccine that is being conducted by US vaccine development company Novavax.
The major trial will take place in sites across the UK including in Northern Ireland and it is anticipated that the first participants will be recruited in Northern Ireland at the start of October.
Up to 350 participants in Northern Ireland will be recruited from the UK Vaccine Registry, which was launched in July. Potential participants who have signed up to the Vaccine Registry to be approached to take part in a vaccine trial will be invited to undergo an assessment to determine whether they are eligible for the trial.
The study aims to recruit adults from all parts of society, especially those who are more likely to benefit from a vaccine including those over 65 year old, those from Black, Asian, and Minority Ethnic backgrounds (BAME), and those who have face to face contact with the public at work e.g. healthcare workers, delivery personnel, transport workers, and those in retail.
Robin Swann, the Minister for Health for Northern Ireland, said in a statement on Monday: “The importance of finding a vaccine to help in the battle against this virus cannot be overstated.
“Despite the magnificent efforts of our health service and the amazing response of society as a whole, we remain on a knife-edge as we seek to protect ourselves and our loved ones.
“As we continue to put in place all possible measures there’s never been a more important time for health research studies into the development of a COVID-19 vaccine. Currently, there are over 30 vaccine trials taking place around the world in an effort to discover as soon as possible which will be safe and effective. It is vital that Northern Ireland joins this important world-wide effort.
Minister Swann added: “I am delighted that the public in Northern Ireland will have the opportunity to take part in this important trial which has the potential to discover a safe and effective vaccine for COVID-19. Already almost 5000 people who are willing to be approached to take part in a trial have signed up to the Vaccine Registry and I would encourage more people here to join today to help this international effort.”
Novavax is conducting its major COVID-19 vaccine trial across the United Kingdom and aims to recruit 10,000 volunteers. Gregory M. Glenn, M.D., President, Research and Development at Novavax, said: “With a high level of SARS-CoV-2 transmission observed and expected to continue in the UK, we are optimistic that this pivotal Phase 3 clinical trial will enroll quickly and provide a near-term view of NVX-CoV2373’s efficacy.
“The data from this trial is expected to support regulatory submissions for licensure in the UK, EU, and other countries.
"We are grateful for the support of the UK Government, including from its Department of Health and Social Care and National Institute for Health Research, to advance this important research.”
Novavax says its upcoming UK Phase 3 clinical trial "is a randomized, placebo-controlled, observer-blinded study to evaluate the efficacy, safety, and immunogenicity of NVX-CoV2373 with Matrix-M in up to 10,000 subjects aged 18 to 84 years.
"Half the participants will receive two intramuscular injections of vaccine comprising 5 µg of protein antigen with 50 µg Matrix‑M adjuvant, administered 21 days apart, while half of the trial participants will receive placebo."
A Northern Ireland COVID-19 Vaccine Research Delivery Group led by the Health and Social Care Research & Development (HSC R&D) Division of the Public Health Agency has been established to coordinate a regional approach to delivering COVID-19 vaccine trials in Northern Ireland. The Novavax trial will be supported in Northern Ireland by the NI Clinical Research Network, which is funded by HSC R&D Division.
Dr. Janice Bailie, Assistant Director HSC R&D of the Public Health Agency in Northern Ireland, said: “COVID-19 vaccine trials are essential to identify which vaccines are both safe and effective so that wide-scale vaccination can start as soon as possible.
“There are different types of vaccines, and we don't know which one will work best to protect people from catching COVID-19. It might be that different vaccines are needed for different groups of people, and it’s only through vaccine trials we will find this out.”
People in Northern Ireland who are interested in participating in the COVID-19 vaccine trial can find more information here and sign up here.


Comments